Immunisation Community of Practice
Welcome to the Immunisation Community of Practice. A community of practice (CoP) is a group of people with a shared passion who come together and learn how to do better. The PHNs Immunisation CoP is your opportunity to get answers, share ideas and build your professional network regarding immunisation.
The PHN Immunisation CoP aims to reduce the incidence of vaccine preventable diseases in the community by providing appropriate and timely information about vaccine preventable diseases and the Immunise Australia Program to immunisation providers and the community and promote the delivery of the National Immunisation Program (NIP).
Immunisation is a simple, safe and effective way of protecting people against harmful diseases that can cause serious health problems in the community. Immunisation not only protects individuals from life-threatening diseases, but also dramatically reduces transmission in the community. The more people who are vaccinated, the fewer opportunities a disease has to spread.
Some of the benefits of joining this Community are:
- 24/7 access to filed Immunisation resources,
- keep current with Immunisation updates,
- brainstorm about Immunisation,
- and network with colleagues passionate about Immunisation.
Welcome to the Immunisation Community of Practice. A community of practice (CoP) is a group of people with a shared passion who come together and learn how to do better. The PHNs Immunisation CoP is your opportunity to get answers, share ideas and build your professional network regarding immunisation.
The PHN Immunisation CoP aims to reduce the incidence of vaccine preventable diseases in the community by providing appropriate and timely information about vaccine preventable diseases and the Immunise Australia Program to immunisation providers and the community and promote the delivery of the National Immunisation Program (NIP).
Immunisation is a simple, safe and effective way of protecting people against harmful diseases that can cause serious health problems in the community. Immunisation not only protects individuals from life-threatening diseases, but also dramatically reduces transmission in the community. The more people who are vaccinated, the fewer opportunities a disease has to spread.
Some of the benefits of joining this Community are:
- 24/7 access to filed Immunisation resources,
- keep current with Immunisation updates,
- brainstorm about Immunisation,
- and network with colleagues passionate about Immunisation.
-
Pertussis cases hit a 35-year high - 9 February 2026
Share Pertussis cases hit a 35-year high - 9 February 2026 on Facebook Share Pertussis cases hit a 35-year high - 9 February 2026 on Twitter Share Pertussis cases hit a 35-year high - 9 February 2026 on Linkedin Email Pertussis cases hit a 35-year high - 9 February 2026 linkPertussis rates are continuing to rise across all Australian state and territories as vaccination numbers fall to the lowest levels in 10 years, according to new data from the Productivity Commission. The latest Report on Government Services (RoGS) shows that in 2024-25 the proportion of children fully immunised under the National Immunisation Program was among the lowest since 2015-16.
-
Laboratory Confirmed Influenza Cases in Australia from 1 January 2026 to 9 February 2026
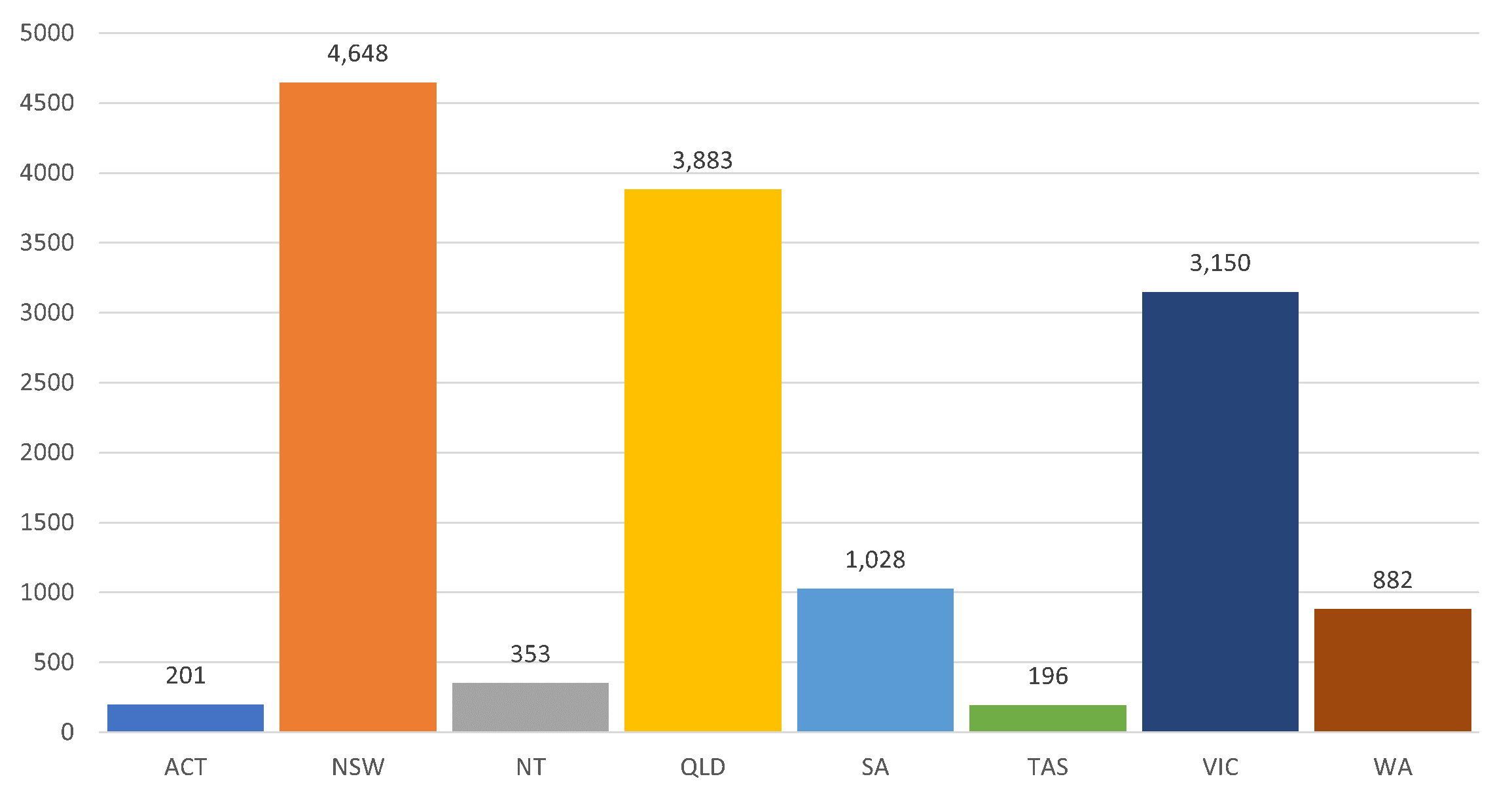
Share Laboratory Confirmed Influenza Cases in Australia from 1 January 2026 to 9 February 2026 on Facebook Share Laboratory Confirmed Influenza Cases in Australia from 1 January 2026 to 9 February 2026 on Twitter Share Laboratory Confirmed Influenza Cases in Australia from 1 January 2026 to 9 February 2026 on Linkedin Email Laboratory Confirmed Influenza Cases in Australia from 1 January 2026 to 9 February 2026 linkThere have been 14,341 notifications of influenza reported to the NNDSS from 1 January 2026 to 9 February 2026.
Data collected from the National Notifiable Diseases Surveillance System (NNDSS).
It is important to note that data reported from the various influenza surveillance systems may not represent an accurate reflection of influenza activity. There may be some delays in the reporting systems. Also, many who become unwell with influenza-like symptoms do not present to their doctor and are not tested for influenza.
Results should be interpreted with caution.
Click here for more statistics on COVID-19, RSV, Influenza, Meningococcal, and Pertussis in Australia. -
Advisory Group Recommendations for Addressing Undervaccination in NIP-Eligible Populations
Share Advisory Group Recommendations for Addressing Undervaccination in NIP-Eligible Populations on Facebook Share Advisory Group Recommendations for Addressing Undervaccination in NIP-Eligible Populations on Twitter Share Advisory Group Recommendations for Addressing Undervaccination in NIP-Eligible Populations on Linkedin Email Advisory Group Recommendations for Addressing Undervaccination in NIP-Eligible Populations linkDespite strong national immunisation programs, Australia continues to experience persistent and, in some cases, worsening undervaccination across several vaccine-preventable diseases. To address this, the Immunisation Coalition has released a new report outlining practical, evidence-aligned recommendations to improve vaccine uptake among populations eligible under the National Immunisation Program (NIP).
The Advisory Group Recommendations for Addressing Undervaccination in NIP-Eligible Populations report focuses on four priority disease areas where coverage gaps remain: COVID-19, influenza, pneumococcal disease and pertussis. These diseases were selected due to declining vaccination rates, ongoing disease burden, and missed opportunities for prevention—particularly among older adults, young children, people with medical risk conditions, pregnant women and residents of aged care facilities.
Developed through expert consensus, the report brings together insights from clinicians, public health specialists, pharmacists, nurses, researchers and industry representatives. Rather than revisiting why vaccination is important, the report concentrates on how to improve delivery, outlining clear, implementable actions that align with the National Immunisation Strategy 2025–2030.
Key themes across the recommendations include:
- Better use of data and the Australian Immunisation Register (AIR) to identify and recall people who are due or overdue for vaccination
- Strengthening vaccination delivery in primary care, aged care and community settings
- Improving access through coordinated programs, funded immunisation coordinators and expanded provider roles
- Providing tailored, fit-for-purpose education for patients, carers and healthcare professionals
- Supporting scalable pilot programs that can be evaluated and expanded nationally
The report is intended to support policymakers, healthcare providers and stakeholders by offering practical solutions that can be actioned now, helping to close coverage gaps and reduce avoidable illness, hospitalisation and death.
Read the full report to explore the recommendations in detail and understand how targeted, system-level changes can strengthen Australia’s immunisation landscape.
-
Chief Medical Officer's updated Measles vaccination advice
Share Chief Medical Officer's updated Measles vaccination advice on Facebook Share Chief Medical Officer's updated Measles vaccination advice on Twitter Share Chief Medical Officer's updated Measles vaccination advice on Linkedin Email Chief Medical Officer's updated Measles vaccination advice linkThe Australian Technical Advisory Group on Immunisation recently updated advice to recommend that infants aged six to 11 months receive an additional dose of a measles-containing vaccine when travelling overseas.
Where an infant aged 6 to 11 months receives an MMR dose before travelling overseas, the dose needs to be repeated. This means that these infants need 2 further doses of measles-containing vaccine. They should receive the next dose of MMR vaccine at 12 months of age or 4 weeks after the 1st dose, whichever is later. They should receive their final dose of measles-containing vaccine as MMRV vaccine at 18 months of age as routinely recommended.
It is not necessary to repeat an early dose if it was given at greater than 11 months but before 12 months of age.
Outbreaks of measles continue in many countries. Australians travelling overseas may be at risk of infection with measles. This includes travelers to popular tourist destinations; for example, periodic outbreaks of measles continue to be reported in Indonesia, including Bali.
Chief Medical Officer Professor Michael Kidd – Measles vaccination advice
-
Utilising AIR10A Reports to Boost Childhood Immunisation Coverage
Share Utilising AIR10A Reports to Boost Childhood Immunisation Coverage on Facebook Share Utilising AIR10A Reports to Boost Childhood Immunisation Coverage on Twitter Share Utilising AIR10A Reports to Boost Childhood Immunisation Coverage on Linkedin Email Utilising AIR10A Reports to Boost Childhood Immunisation Coverage linkAustralia’s childhood vaccination coverage has declined for the third consecutive year—a trend that poses significant risks to public health. Recent data from the Department of Health, Disability and Ageing 2024–25 Annual Report shows that national vaccine coverage targets have not been met for:
- Children at 5 years of age (≥95% target)
- Aboriginal and Torres Strait Islander children between 12 and 15 months of age (≥95% target)
- HPV vaccination of adolescents at 15 years of age (≥90% target by 2030)
As frontline immunisation providers, you play a vital role in reversing this trend. One of the most effective tools available is the AIR10A Overdue Report, which helps practices:
- Identify overdue children: The report lists patients who have missed scheduled immunisations, enabling timely follow-up with families.
- Improve coverage rates: Regular use of AIR10A supports proactive recall and catch-up strategies, reducing the number of overdue children and strengthening community protection.
For eLearning modules on how to request, modify, view and customise AIR reports please visit Australian Immunisation Register (AIR): AIR Reports
By incorporating AIR10A checks into your routine workflow, you can help ensure children receive vaccinations on time and contribute to meeting national coverage targets.
Together, we can make a measurable difference in protecting our communities.
The PHN recommends that practices regularly use Overdue AIR reports to identify children who are overdue for immunisation. Once set up, these reports can become a seamless part of your existing workflows—saving time for your staff while strengthening the quality and continuity of care for your patients.
Need help getting started?
If you’d like more information about requesting AIR reports or assistance with related Quality Improvement activities, please contact our Practice Support team via E: Practicesupport@thephn.com.au or P: 1300 859 028 (option 1) we will be more than happy to provide additional information and support. -
Measles alert for Sydney (12 December 2025)
Share Measles alert for Sydney (12 December 2025) on Facebook Share Measles alert for Sydney (12 December 2025) on Twitter Share Measles alert for Sydney (12 December 2025) on Linkedin Email Measles alert for Sydney (12 December 2025) linkNSW Health is advising people to be alert for signs and symptoms of measles after being notified of a confirmed case who was infectious while visiting several locations in Sydney.
The case recently returned from South-East Asia, where there are ongoing outbreaks of measles in several countries including Indonesia.
People who attended the following locations should watch for the development of symptoms. These locations do not pose an ongoing risk.
- Sydney Metro and T4 Train Lines – Macquarie University Station to Edgecliff Station via Martin Place Station at various dates and times between Wednesday 3 and Saturday 8 December
- Gran Torino Restaurant, 24 Bay St, Double Bay at various dates and times between Wednesday 3 and Saturday 6 December
- Macquarie Centre Food Court, Waterloo Rd Macquarie Park, between 3:30 and 5:30 on Friday 5 and Saturday 6 December
The full list of locations and times is on the NSW Health website.
Northern Sydney Local Health Public Health Unit Director, Dr Michael Staff, said anyone who visited the above locations at those times should monitor for symptoms.
“Measles is a vaccine preventable disease that is spread through the air when someone who is infectious coughs or sneezes," Dr Staff said.
“Symptoms to watch out for include fever, sore eyes, runny nose and a cough, usually followed three or four days later by a red, blotchy rash that spreads from the head and face to the rest of the body.
“It can take up to 18 days for symptoms to appear after an exposure, so it's important for people who visited these locations to look out for symptoms up until 27 December.
“If they develop symptoms, they should call ahead to their GP or emergency department to ensure they do not spend time in the waiting room with other patients."
“We want to remind the community to make sure they are up to date with their vaccinations. The measles vaccine can prevent the disease even after exposure, if given early enough."
“Anyone born after 1965 needs to ensure they have had two doses of measles vaccine. This is especially important before overseas travel, as measles outbreaks are occurring in several regions of the world at the moment," Dr Staff said.
The measles-mumps-rubella (MMR) vaccine is safe and effective, and is given free for children at 12 and 18 months of age. It is also free in NSW for anyone born after 1965 who hasn't already had two doses.
Children under the age of 12 months can have a dose of MMR from six months of age if they are travelling overseas. Parents should consult their GP.
People who are unsure of whether they have had two doses should get a vaccine, as additional doses are safe. This is particularly important prior to travel. MMR vaccine is available from GPs (all ages) and pharmacies (people over 5 years of age).
For more information on measles, view the measles factsheet .
If you, or a loved one, is experiencing measles symptoms, or have questions about measles, please call your GP or Healthdirect on 1800 022 222.
-
Australian Technical Advisory Group on Immunisation (ATAGI) statement on the importance and safety of Hepatitis B vaccine a birth
Share Australian Technical Advisory Group on Immunisation (ATAGI) statement on the importance and safety of Hepatitis B vaccine a birth on Facebook Share Australian Technical Advisory Group on Immunisation (ATAGI) statement on the importance and safety of Hepatitis B vaccine a birth on Twitter Share Australian Technical Advisory Group on Immunisation (ATAGI) statement on the importance and safety of Hepatitis B vaccine a birth on Linkedin Email Australian Technical Advisory Group on Immunisation (ATAGI) statement on the importance and safety of Hepatitis B vaccine a birth linkATAGI has released advice regarding the importance and safety of Hepatitis B vaccine at birth. The statement is available on the Department of Health, Disability and Ageing website.
-
Japanese Encephalitis vaccination - Important information
Share Japanese Encephalitis vaccination - Important information on Facebook Share Japanese Encephalitis vaccination - Important information on Twitter Share Japanese Encephalitis vaccination - Important information on Linkedin Email Japanese Encephalitis vaccination - Important information linkOn 4th December, NSW Health issued a Media Release regarding the first 2025-2026 summer season detection of Japanese Encephalitis virus in mosquitoes in rural Victoria.
- NSW residents urged to take care this mosquito season [4 December 2025]
NSW Health's Acting Director of Health Protection, Dr Stephen Conaty, said these detections so early in the summer season are concerning.
“Mosquito numbers will likely increase with warmer weather, and we remind everyone in NSW to protect themselves against mosquito bites, which can cause diseases such as Japanese Encephalitis and Murray Valley Encephalitis, and infection with Kunjin virus, Ross River virus and Barmah Forest virus," Dr Conaty said.
NSW Health have also sent a Provider Alert to all Immunisation providers in NSW asking them to be alert to the increased risk of mosquito borne diseases and promote vaccination.
For information on eligibility criteria for funded JE vaccine https://www.health.nsw.gov.au/Infectious/jev/Pages/vaccination.aspx
Provider tookit https://www.health.nsw.gov.au/Infectious/jev/Documents/jev-vaccination-toolkit.pdf
For information on eligibility criteria for funded JE vaccine https://www.health.nsw.gov.au/Infectious/jev/Pages/vaccination.aspx
-
Vaccine recommendations for pregnant women – a guide for health professionals
Share Vaccine recommendations for pregnant women – a guide for health professionals on Facebook Share Vaccine recommendations for pregnant women – a guide for health professionals on Twitter Share Vaccine recommendations for pregnant women – a guide for health professionals on Linkedin Email Vaccine recommendations for pregnant women – a guide for health professionals linkThe NCIRS guide on vaccine recommendations for pregnant women provides current vaccine recommendations for pregnant women in Australia. It summarises the latest recommendations, safety information and key clinical considerations based on gestational age in one easy-to-use resource. Vaccine recommendations for pregnant women – a guide for health professionals
-
Measles vaccination – a guide for immunisation providers
Share Measles vaccination – a guide for immunisation providers on Facebook Share Measles vaccination – a guide for immunisation providers on Twitter Share Measles vaccination – a guide for immunisation providers on Linkedin Email Measles vaccination – a guide for immunisation providers linkThis updated measles vaccination guide provides clear, easy-to-follow guidance for immunisation providers. The resource simplifies assessment by outlining measles vaccination requirements based on age and previous number of doses. Measles vaccination – a guide for immunisation providers | NCIRS
Who's Listening
-

Phone 0409 148 062 Email kwisemantel@thephn.com.au
Key Dates and Education
Documents
-
 2025 Adult Pneumococcal Vaccine Recall Program
2025 Adult Pneumococcal Vaccine Recall Program
-
 Immunisation and Quality Improvement
Immunisation and Quality Improvement
-
 12 Month Quality Improvement Record Template (BLANK) (151 KB) (docx)
12 Month Quality Improvement Record Template (BLANK) (151 KB) (docx)
-
 One PIP QI Quarter Quality Improvement Record Template (BLANK) (145 KB) (docx)
One PIP QI Quarter Quality Improvement Record Template (BLANK) (145 KB) (docx)
-
 Immunisation QI Toolkit (459 KB) (pdf)
Immunisation QI Toolkit (459 KB) (pdf)
-
 Immunisation PDSA Example: Influenza Vaccination (205 KB) (pdf)
Immunisation PDSA Example: Influenza Vaccination (205 KB) (pdf)
-
 QI Activity AIR10A reports - followup overdue children (649 KB) (pdf)
QI Activity AIR10A reports - followup overdue children (649 KB) (pdf)
-
 Practice Incentive Program Quality Improvement and CAT Plus (5.2 MB) (pdf)
Practice Incentive Program Quality Improvement and CAT Plus (5.2 MB) (pdf)
-
 AIR010A Actions for overdue children.pdf (318 KB) (pdf)
AIR010A Actions for overdue children.pdf (318 KB) (pdf)
-
 Childhood Vaccines AIR010A report parameters.pdf (291 KB) (pdf)
Childhood Vaccines AIR010A report parameters.pdf (291 KB) (pdf)
-
-
 Cold Chain Management
Cold Chain Management
-
 Cold Chain PHN doc.pdf (286 KB) (pdf)
Cold Chain PHN doc.pdf (286 KB) (pdf)
-
 Cold Chain Breach Protocol Poster (188 KB) (pdf)
Cold Chain Breach Protocol Poster (188 KB) (pdf)
-
 Cold Chain Breach Reporting Form (338 KB) (pdf)
Cold Chain Breach Reporting Form (338 KB) (pdf)
-
 NSW Health Cold Chain Toolkit (606 KB) (pdf)
NSW Health Cold Chain Toolkit (606 KB) (pdf)
-
 Strive for 5 Vaccine Fridge Temperature Chart Resource (129 KB) (pdf)
Strive for 5 Vaccine Fridge Temperature Chart Resource (129 KB) (pdf)
-
 National Vaccine Storage Guidelines: Strive for 5 (3rd ed) (6.05 MB) (pdf)
National Vaccine Storage Guidelines: Strive for 5 (3rd ed) (6.05 MB) (pdf)
-
-
 Immunisation and the MBS
Immunisation and the MBS
-
 Immunisation AIR and PRODA
Immunisation AIR and PRODA
-
 Services Australia: AIR-010A Due and Overdue Immunisation by Practice Report (1.38 MB) (pdf)
Services Australia: AIR-010A Due and Overdue Immunisation by Practice Report (1.38 MB) (pdf)
-
 Services Australia: Australian Immunisation Register Data Quality User Guide (991 KB) (pdf)
Services Australia: Australian Immunisation Register Data Quality User Guide (991 KB) (pdf)
-
 Department of Health: Australian Immunisation Register (AIR) Fact sheet (274 KB) (pdf)
Department of Health: Australian Immunisation Register (AIR) Fact sheet (274 KB) (pdf)
-
 AIR access via PRODA PHN Doc (834 KB) (pdf)
AIR access via PRODA PHN Doc (834 KB) (pdf)
-
 Recording vaccinations given overseas on the Australian Immunisation Register (AIR) PHN Doc (168 KB) (pdf)
Recording vaccinations given overseas on the Australian Immunisation Register (AIR) PHN Doc (168 KB) (pdf)
-
 Requesting and viewing the COVID-19 Vaccination Status Report (AIR42A) PHN Doc (526 KB) (pdf)
Requesting and viewing the COVID-19 Vaccination Status Report (AIR42A) PHN Doc (526 KB) (pdf)
-
-
 Immunisation - Nurses
Immunisation - Nurses
-
 How to request an AIR 10A report.pdf (386 KB) (pdf)
How to request an AIR 10A report.pdf (386 KB) (pdf)
-
 Immunisation contacts November 2023.docx (137 KB) (docx)
Immunisation contacts November 2023.docx (137 KB) (docx)
-
 AIR-010A-Report-User-Guide-Nov-2020-V1.0.pdf (1.41 MB) (pdf)
AIR-010A-Report-User-Guide-Nov-2020-V1.0.pdf (1.41 MB) (pdf)
Important Links
- The Australian Immunisation Handbook
- NSW Health Immunisation Programs
- National Centre for Immunisation Research and Surveillance Australia
- Central Coast HealthPathways
- Hunter New England HealthPathways
- Central Coast LHD Immunisation
- HNE Health Immunisation
- Sharing Knowledge About Immunisation (SKAI)